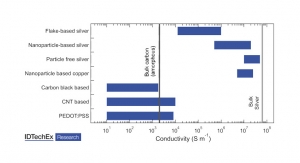
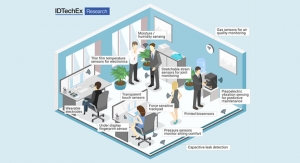
Dave Savastano, Editor03.09.15
The market for flexible and printed electronics (PE) spans a wide assortment of industries, from sensors to wearables, displays to photovoltaics and more. The goal has been to develop products that reach the mainstream market. There are notable successes like medical sensors – most notably glucose strips – which have become a billion dollar business.
There are plenty of new products heading from the labs to pilot production and commercialization, and in talking with industry leaders throughout the value chain, there are quite a few new applications that stand out.
“Printed electronics is an enabler for wearable technology,” said IDTechEx CEO Raghu Das. “An example on the market includes the baby monitoring system using ECM ink sold by Babies R Us, the TempTraq announcement from Blue Spark, the heated and sound-based jacket shown at our event by Teiimo and new materials enabling this, e.g. from DuPont MCM.”

The iilation Jacket
According to Teiimo, the iilation Jacket is the first leather jacket that provides heat to the user, while also serving as a Bluetooth hands-free calling device. In addition, the iilation jacket plays music and can charge cell phones.
One growth area is product security. Pharmaceutical companies and consumers alike are deeply concerned about counterfeit drugs, which imperil people’s health. Thin Film Electronics (Thinfilm) is working with Global Factories, the global market leader in the field of medicine pouch verification, on an authentication solution using Thinfilm Memory.
“Refill authentication is an important application that is using printed electronics,” said Jennifer Ernst, Thinfilm’s chief strategy officer. “For example, Global Factories recently implemented a printed electronics solution for pharmaceutical security. Global Factories is using printed electronics for an authentication solution in its next-generation medicine pouch verification system, the Vandenbrink Blister Packaging Machine (VBM).”
Ernst said that the VBM automates the filling of unit- and multi-dose blister trays. Each tray contains pouches that are filled with a combination of medication to be taken at prescribed times. The separate pouches help patients take the correct dosage and combination of medication throughout the day.
“Global Factories is using Thinfilm Memory labels to ensure that only safe and qualified materials are being used in their systems,” Ernst said. “The VBM reads the memory label when it is inserted into the dispensing unit. Once it has verified that authorized trays are being used, the machine is unlocked and dispenses the pre-set limit of refills to the pouches. By using printed electronics technology, Global Factories is able to implement a secure, cost-effective, highly scalable and easy-to-integrate solution.”

Kent Displays Boogie Board Sync eWriter.
Kent Displays, Inc. has received critical and commercial success with its line of flexible Boogie Board eWriters. Boogie Board utilizes a pressure sensitive LCD writing surface that allows the user to draw or write memos, which they can store or send. Kent Displays received the Best Commercialization Award from IDTechEx during Printed Electronics USA 2014, and Das said it is an excellent example of a product that has achieved commercial success.
“The Boogie Board from Kent Displays has been commercially successful,” Das said. “It is a great example of a display company moving downstream to make a complete product.”
Dr. Joshua Windmiller, Electrozyme LLC’s CEO, noted that products leveraging flexible and printed electronics (FPE) are already commercially available.
“From a consumer perspective, I have had the distinct opportunity to advance printed epidermal electrochemical biosensors from conception at my benchtop to integratable sub-systems that are currently being licensed to major Fortune 500 corporations,” Dr. Windmiller said. “These biosensors, which are fabricated via screen printing methods, feature the unique ability to quantify electrolyte concentration in the sweat and hence electrolyte loss during activity. This information can be leveraged by the user to gain insight into an appropriate personalized hydration regimen, such as when the consumption of a sports drink rather than water would be beneficial. In the healthcare domain, the ability to monitor chloride ions using this same sweat electrolyte quantification paradigm has the potential to disrupt current practices in cystic fibrosis that exploit a six-decade-old diagnostic test plagued by a high level of inaccuracy and false positives.
“One of the most well-recognized applications of FPE include RFID and NFC tags, which are moving to greater levels of sophistication and complexity aided by advances in hybrid systems integration,” Dr. Windmiller added. “Displays and lighting represent additional and widely deployed embodiments of FPE technology, with products featuring improved color rendition, contrast and efficiency than their solid-state counterparts. Body-worn sensors leveraging FPE are a recent addition to the healthcare space, albeit applications have been limited in scope and utility. Based on the current research pipeline, I would venture to say that, over the next decade, we will witness increased proliferation of FPE in consumer-level products, with many niche applications of the technology going mainstream.”
Sensoria CEO Davide Vigano noted that Sensoria is seeing growth in the field of flexible and printed wearable electronics.
“It is a journey,” Vigano said. “We use flex in our Sensoria anklet today. However we need truly flexible/printed and high density electronics. The most interesting project that we have worked on is combining our own textile pressure sensors with flexible electronics in our Sensoria sock. Our truly wearable technology is now reaching commercialization phase.”
Dr. Laura López, head of scientific valorisation at CETEMMSA, said CETEMMSA is seeing increasing interest in the field of flexible electronics. “We are now working on exciting projects directly with companies developing products with PE very close to market,” Dr. Lopez added.
CETEMMSA has some successes already in the field of wearables. One example is the TOF-Cuff Medical Device, invented by Dr. Josep Rodiera, designed by CETEMMSA and manufactured by RGB Medical Devices Company, in Madrid. TOF-Cuff tests blood pressure and neuromuscular transmission monitoring under anesthesia in just one device.
Dr. Malcolm G. Keif, professor and graduate coordinator printed electronics and functional imaging for Cal Poly State University’s Graphic Communication Department, said that flexible hybrid electronics open up new opportunities for producers and consumers.

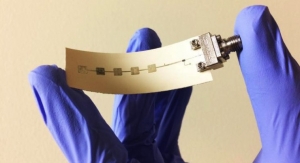
TempTraq thermometer.
“The key is flexibility and lightweighting,” Dr. Keif added. “A perfect example of this is TempTraq, Blue Spark Technologies’ new single use thermometer. When a baby is sick, the parent doesn’t want to disturb a sleeping child to take the baby’s temperature. The conformable, lightweight patch allows the parent to monitor the baby’s temperature continually. This is where the near term growth is. We will likely see many more products in the near future that integrate conventional with printed forms to make new lightweight conformable applications.”
TempTraq is an adhesive bandage powered by Blue Spark Technologies’ printed flexible batteries. Introduced at the 2015 Consumer Electronics Show and pending FDA 510(k) premarket review, TempTraq is a wearable intelligent Bluetooth thermometer in the form of a soft patch that continuously and comfortably monitors body temperature for 24 hours. It includes a free downloadable Apple or Android compatible app, which allows parents to review data and receive alerts, up to 40 feet away, and covers historical and real time data.
“TempTraq allows parents to keep a close watch on their child’s temperature without having to continually disturb them,” said John Gannon, president and CEO of Blue Spark Technologies.
There are a wide range of new applications that hold promise for flexible and printed electronics. LOPE general chair Wolfgang Mildner said that it would be unfair to focus on just one of the many projects.
“A good option is always to listen to the opinion of the jury of the LOPEC competitions, for example, the OE-A Demonstrator Competition,” Mildner said. “In 2014, there were quite a few interesting demonstrators in the four categories we have. The winners were a multi-touch sensor for smart phones, a flexible timer for rough environments, fully printed batteries and several concepts dealing with organic and printed electronics in packaging. These projects show the great potential of the technology.”
There are plenty of new products heading from the labs to pilot production and commercialization, and in talking with industry leaders throughout the value chain, there are quite a few new applications that stand out.
“Printed electronics is an enabler for wearable technology,” said IDTechEx CEO Raghu Das. “An example on the market includes the baby monitoring system using ECM ink sold by Babies R Us, the TempTraq announcement from Blue Spark, the heated and sound-based jacket shown at our event by Teiimo and new materials enabling this, e.g. from DuPont MCM.”
The iilation Jacket
One growth area is product security. Pharmaceutical companies and consumers alike are deeply concerned about counterfeit drugs, which imperil people’s health. Thin Film Electronics (Thinfilm) is working with Global Factories, the global market leader in the field of medicine pouch verification, on an authentication solution using Thinfilm Memory.
“Refill authentication is an important application that is using printed electronics,” said Jennifer Ernst, Thinfilm’s chief strategy officer. “For example, Global Factories recently implemented a printed electronics solution for pharmaceutical security. Global Factories is using printed electronics for an authentication solution in its next-generation medicine pouch verification system, the Vandenbrink Blister Packaging Machine (VBM).”
Ernst said that the VBM automates the filling of unit- and multi-dose blister trays. Each tray contains pouches that are filled with a combination of medication to be taken at prescribed times. The separate pouches help patients take the correct dosage and combination of medication throughout the day.
“Global Factories is using Thinfilm Memory labels to ensure that only safe and qualified materials are being used in their systems,” Ernst said. “The VBM reads the memory label when it is inserted into the dispensing unit. Once it has verified that authorized trays are being used, the machine is unlocked and dispenses the pre-set limit of refills to the pouches. By using printed electronics technology, Global Factories is able to implement a secure, cost-effective, highly scalable and easy-to-integrate solution.”
Kent Displays Boogie Board Sync eWriter.
“The Boogie Board from Kent Displays has been commercially successful,” Das said. “It is a great example of a display company moving downstream to make a complete product.”
Dr. Joshua Windmiller, Electrozyme LLC’s CEO, noted that products leveraging flexible and printed electronics (FPE) are already commercially available.
“From a consumer perspective, I have had the distinct opportunity to advance printed epidermal electrochemical biosensors from conception at my benchtop to integratable sub-systems that are currently being licensed to major Fortune 500 corporations,” Dr. Windmiller said. “These biosensors, which are fabricated via screen printing methods, feature the unique ability to quantify electrolyte concentration in the sweat and hence electrolyte loss during activity. This information can be leveraged by the user to gain insight into an appropriate personalized hydration regimen, such as when the consumption of a sports drink rather than water would be beneficial. In the healthcare domain, the ability to monitor chloride ions using this same sweat electrolyte quantification paradigm has the potential to disrupt current practices in cystic fibrosis that exploit a six-decade-old diagnostic test plagued by a high level of inaccuracy and false positives.
“One of the most well-recognized applications of FPE include RFID and NFC tags, which are moving to greater levels of sophistication and complexity aided by advances in hybrid systems integration,” Dr. Windmiller added. “Displays and lighting represent additional and widely deployed embodiments of FPE technology, with products featuring improved color rendition, contrast and efficiency than their solid-state counterparts. Body-worn sensors leveraging FPE are a recent addition to the healthcare space, albeit applications have been limited in scope and utility. Based on the current research pipeline, I would venture to say that, over the next decade, we will witness increased proliferation of FPE in consumer-level products, with many niche applications of the technology going mainstream.”
Sensoria CEO Davide Vigano noted that Sensoria is seeing growth in the field of flexible and printed wearable electronics.
“It is a journey,” Vigano said. “We use flex in our Sensoria anklet today. However we need truly flexible/printed and high density electronics. The most interesting project that we have worked on is combining our own textile pressure sensors with flexible electronics in our Sensoria sock. Our truly wearable technology is now reaching commercialization phase.”
Dr. Laura López, head of scientific valorisation at CETEMMSA, said CETEMMSA is seeing increasing interest in the field of flexible electronics. “We are now working on exciting projects directly with companies developing products with PE very close to market,” Dr. Lopez added.
CETEMMSA has some successes already in the field of wearables. One example is the TOF-Cuff Medical Device, invented by Dr. Josep Rodiera, designed by CETEMMSA and manufactured by RGB Medical Devices Company, in Madrid. TOF-Cuff tests blood pressure and neuromuscular transmission monitoring under anesthesia in just one device.
Dr. Malcolm G. Keif, professor and graduate coordinator printed electronics and functional imaging for Cal Poly State University’s Graphic Communication Department, said that flexible hybrid electronics open up new opportunities for producers and consumers.
TempTraq thermometer.
TempTraq is an adhesive bandage powered by Blue Spark Technologies’ printed flexible batteries. Introduced at the 2015 Consumer Electronics Show and pending FDA 510(k) premarket review, TempTraq is a wearable intelligent Bluetooth thermometer in the form of a soft patch that continuously and comfortably monitors body temperature for 24 hours. It includes a free downloadable Apple or Android compatible app, which allows parents to review data and receive alerts, up to 40 feet away, and covers historical and real time data.
“TempTraq allows parents to keep a close watch on their child’s temperature without having to continually disturb them,” said John Gannon, president and CEO of Blue Spark Technologies.
There are a wide range of new applications that hold promise for flexible and printed electronics. LOPE general chair Wolfgang Mildner said that it would be unfair to focus on just one of the many projects.
“A good option is always to listen to the opinion of the jury of the LOPEC competitions, for example, the OE-A Demonstrator Competition,” Mildner said. “In 2014, there were quite a few interesting demonstrators in the four categories we have. The winners were a multi-touch sensor for smart phones, a flexible timer for rough environments, fully printed batteries and several concepts dealing with organic and printed electronics in packaging. These projects show the great potential of the technology.”